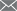Liu Ruizhi 1, Ma Jiaomei 1, Tao Congxi 1, Chen Xinzhi 2, Duan Mingzi 2
(1. Sinoma International Research Institute, Tianjin 300400; 2. Guangdong Wanxun Technology Development Co., Ltd., Guangzhou 510075)
Abstract: A comprehensive thermal analysis experiment was conducted on the chemical constituents of sulfur-fixing agents developed by Sinoma International Research Institute, School of Materials Science and Engineering of South China University of Technology and Guangdong Wanxun Technology Development Co., Ltd., and the absorption of various chemical components in sulfur-fixing agents was compared. The rate of SO2, combined with the test results, optimized the formulation and the incorporation position, and carried out industrial experiments in the South China ZS Cement Plant to achieve the desulfurization effect.
Key words: SO2; cement; comprehensive thermal analysis; sulfur-fixing agent
CLC number: TQ172.9 Document code: A Article ID: 1671-8321 (2016) 08-0079-03
0 Preface
Compared with power plants, the new dry process cement production line is equivalent to a natural desulfurization tower. In the cement production process, there is a large amount of CaO capable of absorbing SO2, which can absorb a large part of SO2 to form sulfate and solidify in cement silicate minerals along with clinker. Discharge the cement kiln system. However, with the increasingly strict environmental protection requirements of the country and the industry, SO2 emission reduction is one of the most important topics in cement technology research. The impact of SO2 emissions in cement plants mainly comes from raw materials, especially organic sulfur or FeS2 in limestone, which forms SO2 discharge preheater in the second and third stage cyclone as the raw material is fed into the preheater; The sulphate in the fuel and the sulphur in the fuel, because of the opportunity to contact with CaO, or the formation of sulphate with the cement clinker, or the pyrolysis of the low-temperature internal circulation, or the formation of local crust in the smoke chamber, etc., will not enter Flue gas affects sulfur emissions. Therefore, the mechanism of sulfur emission and how to carry out desulfurization with high efficiency and low cost have always been the focus of the company's research.
In response to this problem, we conducted research with the School of Materials Science and Engineering of South China University of Technology and Guangdong Wanxun Technology Development Co., Ltd. The analysis believes that adding sulfur-fixing powder to the kiln hoist is an important means to solve the desulfurization. The sulfur-fixing powder is mainly composed of a calcium-based powder and is further processed by a plurality of metal oxides or compounds including rare earths. Here, we used a thermal analysis instrument to carry out a comprehensive thermal analysis experiment on the sulfur-fixing powder, and compared the rate of absorption of SO2 by each chemical component in the sulfur-fixing agent. The formulation was optimized according to the test results, and good test results were obtained.
1 Catalytic test of SO2 absorption by different catalysts in laboratory
1.1 Experimental instruments and conditions
We use the German NETZSCH thermal analysis instrument (see Figure 1). The thermal analysis experiment uses a thermogravimetric instrument to test the sample. The weight of the test sample changes with temperature at the programmed temperature. This includes the sample at The program controls the process of exothermic or endothermic temperatures. The following are the conditions required for the test:
Analytical instrument: NETZSCH STA 409PC;
Atmosphere: air;
Flow rate: O2 13 ml/min Purge N2: 40 ml/min Protect N2: 10 ml/min.

Figure 1 NETZSCH STA 409PC thermal analysis instrument
1.2 Catalytic test of SO2 absorption by different catalysts
The laboratory prepares SO2 by reacting FeS2 with O2 to form Fe3O4 and SO2. Then use Ca(OH)2 to react with SO2 to absorb SO2. During this period, different components of the catalyst, such as titanium dioxide, cerium oxide, lithium hydroxide, magnesium oxide, etc., are added in an amount of 10% to verify the catalysts of different components. The absorption efficiency of Ca(OH)2 for SO2 absorption. The following is the chemical equation for this reaction:
3FeS2+8O2=Fe3O4+6SO2
6Ca(OH)2+6SO2=6CaSO3+6H2O
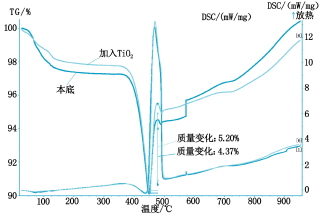
The thermal analysis curves are shown in Figures 2 to 5, respectively.
It can be seen from Fig. 2 that the absorption rate of SO2 by calcium hydroxide is 4.37% when no catalyst is added, and the absorption rate of SO2 is 5.2% by adding titanium dioxide, because titanium dioxide is a rare earth oxide, which accelerates The absorption efficiency of calcium hydroxide on SO2; it can be seen from Fig. 3 that the addition of cerium oxide further promotes its absorption of SO2 at a rate of 7.24%, and the reaction mechanism is the same as before; Figure 4 shows that lithium hydroxide The absorption of SO2 is greatly improved at a rate of 20.13%. This is because lithium hydroxide itself acts as a very active base, on the one hand, it has an absorption effect on SO2, on the other hand, it also causes the absorption of SO2 by calcium hydroxide. Catalytic action; it can be seen from Figure 5 that magnesium oxide has little catalytic effect on the absorption of SO2.
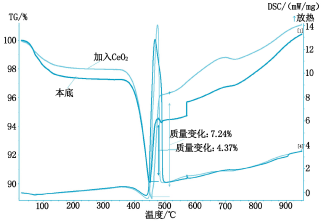
Figure 3 CeO2 absorption SO2 thermal analysis curve
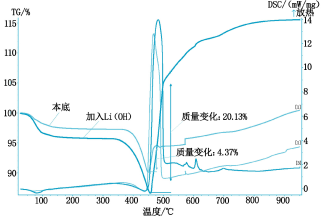
Fig. 4 Thermal analysis curve of Li(OH)2 absorbed SO2
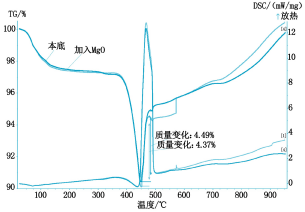
Fig. 5 Graph of thermal analysis of MgO absorption SO2
It can be seen from the above experiments that titanium dioxide, cerium oxide, lithium hydroxide and the like can all catalyze the absorption of sulfur dioxide by calcium hydroxide. The industrial test plan is based on the sulfur-fixing powder as the main component, coupled with the liquid spray synergy, which will greatly improve the absorption efficiency of SO2. The data show that water is also a catalyst for calcium hydroxide. We have done a desulfurization industrial test at the ZS Cement Plant and achieved breakthrough results, as described below.
2 Desulfurization industrial test
2.1 Introduction to the production line
ZS Cement Plant is a new dry-process cement clinker production line of 5 000t/d. It was completed and put into production in 1989. The production line has a medium-loading lifting and drying raw material grinding machine, a controlled flow raw material homogenization library, and a wind-swept ball mill (coal). The firing system uses a five-stage dual series preheater, SLC spray off-line decomposing furnace and a Fullux grate cooler and a pre-decomposition kiln system of φ4.75m×75m rotary kiln; equipped with advanced X-ray fluorescence analyzer and Dust facilities, etc. The limestone used in the cement plant is high-sulfur limestone with a sulfur content of 0.2% to 0.4%. Therefore, the SO2 emission concentration in the cement kiln flue gas exceeds the standard (600mg/Nm3 when grinding is stopped, and 380mg/Nm3 when grinding is started). Many). According to the relevant national standards, “Notice on Execution of Special Emission Limits of Air Pollutants”, (Notified by the Ministry of Environmental Protection, No. 14 of 2012), “Emission Standards for Air Pollutants in Cement Industry” (GB 4915-2013), “Coal Kiln Synergy Disposal Waste Pollution Control Standard (GB30485-2013), which stipulates that the SO2 emission concentration in cement kiln flue gas is ≤200mg/Nm3, and the discharge per unit product needs to be less than 0.6kg/t. In January 2014, the local standard of Guangdong Province, “Emission Standards for Air Pollutants in Cement Industry Enterprises” (DB44/818-2010) stipulated that the concentration of SO2 in cement kiln flue gas is ≤100mg/Nm3.
2016.8 CHINA CEMENT 119
Applied Research Research & Application The concentration of SO2 in the flue gas of the plant is 6 times that of the provincial standard. Therefore, when the factory agent is 0.1% and the water agent is 0.5m3/h, the SO2 emission value reaches the urgent requirement that the desulfurization is reduced to 100mg/ Below Nm3.
2.2 technical solutions
In this test, due to the small background emission of the plant (about 380mg/Nm3 when grinding), the following technical solutions were proposed according to the laboratory test results: (1) 2% of the sulfur-fixing agent was gradually reduced to 0.1%. And the corresponding liquid addition amount is reduced from 1t / h to 0.5t / h; (2) adjustment of the addition position: into the kiln hoist; (3) the formula is based on calcium, adding rare earth elements such as metal oxides . Figure 6 shows the location of the sulfur-fixing agent added to the ZS plant.
Figure 6 ZS cement plant added sulfur-fixing agent position
Experimental procedure
The test adopts powder-based agent, powder and water agent are combined, and different dosages of powder-fixing agent are added according to the amount of raw material feeding. At the same time, different flow rates are sprayed in the secondary cylinder of the preheater to the riser of the first-stage cylinder. Liquid desulfurizer, adjust the ratio of powder to liquid for different SO2 background emission values, conduct orthogonal test, monitor SO2 emission in cement kiln flue gas in real time, and finally determine the optimal and reasonable desulfurizer dosage. Compared, to ensure the realization of SO2 emission standards in the cement kiln flue gas. In the test, the amount of the powder sulfur-fixing agent is 2%, 1%, 0.5%, 0.1% of the raw material feeding amount, and the dosage is relative to the percentage of the raw material, and the liquid desulfurizing agent is used in an amount of 0.5 m3/h. ~2.0m3/h continuously adjustable.
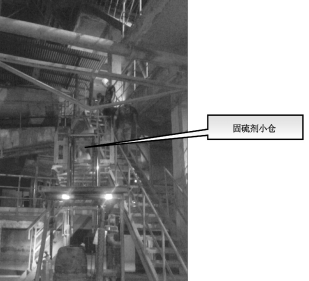
Figure 7 SO2 emission values before and after the test at ZS Cement Plant (mg/Nm3)
Figure 7 shows the SO2 emission value (mg/Nm3) before and after the test at ZS Cement Plant. It can be seen from the figure that before the sulfur-fixing agent is added, the background emission value of SO2 at the time of grinding is about 400 mg/Nm3, and the SO2 at the time of grinding is stopped. The emission value is about 600mg/Nm3, and the SO2 emission value before and after 23:00 is less than 100mg/Nm3, which is the initial attempt of the day before the official test; and the formal test is carried out after 15:00 the next day, after continuous adjustment, And after the best combination of powder and water, four hours from 18:00 to 22:00, when the powder
Figure 8 ZS emission value before and after the test of ZS cement plant (mg/Nm3) (enlarged view)
2.4 Desulfurization Industry Experiment Summary
From the desulfurization industry test of the plant, this system is feasible, can be reduced from the background discharge of 600mg/Nm3 within 10min to below 100mg/Nm3, and can continue to operate. At present, ZS Cement Plant has been continuously using sulfur-fixing powders and water-based agents. The cost is estimated based on the use of more than half a year. The cost of desulfurization of tons of clinker is about 2 yuan, which can maintain stable discharge.
3 conclusions
(1) Rare earth oxides improve the catalytic effect of calcium on SO2, and can be used according to the ingredients in the factory.
(2) With the solid sulfur powder as the main body and the synergistic action of the liquid spray, the absorption efficiency of SO2 will be greatly improved.
(3) Desulfurization industrial test results show that this desulfurization scheme can be reduced from 100mg/Nm3 in the background to less than 100mg/Nm3 in 10min, and it can continue to operate; the cost of desulfurization of tons of clinker is about 2 yuan, the cost is low, and the effect is good. .